Correctly Understand the Self Discharge of Battery

Are you using or buying batteries? It's important to understand a battery's self-discharge—the process by which the energy stored in a battery decreases even when it's not in use.
Don't worry if you don't know what this means, we'll dive into all aspects related to self-discharge and how it can affect your use.
In today's blog post, we'll discuss what causes batteries to self-discharge, understand why this phenomenon is necessary, so we can take appropriate steps to extend lithium battery cycle life .
So let's start to better understand how self-discharge works!
What is Self Discharge of Battery?
Theoretically, the batteries do not react with each other when they are not in use, and the redox reaction only occurs when the appliance is connected to the battery.
However, in real life, ordinary batteries, including dry batteries (alkaline), will produce chemical reactions inside the battery. That is, there is no connection between the electrodes, and a very small amount of chemical substances in the battery will react.
Many see circuits as just one possible way to direct the flow of electrons to their desired location. There are also things like degraded electrolytes, leaks, etc., but the most prominent ones are due to the chemistry of the battery itself.
These internal reactions reduce the battery's stored charge, gradually reducing the battery's capacity. This phenomenon is called self-discharge.
The Major Causes of Self Discharging in Battery
(1) 3 Main points
- After the battery is stored for a long time, the sulfuric acid will sink, causing a potential difference between the upper and lower plates, and the electrolyte overflowed from the battery will accumulate on the surface of the battery cover, and the positive and negative polarities will form channels, resulting in self-discharge.
- The electrolyte and battery plate materials are impure, and a potential difference is formed between impurities and the plate and between different impurities deposited on the plate, and partial discharge is generated through the electrolyte.
- The active material of the battery plate falls off, and the lower part of the deposit is too much, resulting in a short circuit of the plate, and the upper and lower layers of the battery electrolyte, resulting in self-discharge.
(2) Physical factors
Why does a battery placed in a circuit breaker lose its charge? Physical factors mainly come from the loss of electrochemical substances inside the battery and internal short circuits.
The loss of battery material is irreversible and will cause the loss of battery capacity, but loss refers to the embodiment of capacity recovery performance. For example, the power loss caused by a short circuit will consume current power, and the capacity will not be affected by this part of the reaction.
The sum of capacity loss (irreversible) and simple power loss (reversible) is the self-discharge amount.
- Side reactions of electrochemical materials
Material side reactions mainly occur in three aspects: positive electrode material, negative electrode material and electrolyte.
| Material | Material Side Reactions |
| Cathode material | It is mainly a variety of lithium compounds, which always have a slight reaction with the electrolyte, and the degree of reaction is different under different environmental conditions.
The positive electrode material reacts with the electrolyte to form insoluble products, making the reaction irreversible. The positive electrode material involved in the reaction loses its original structure, and the battery loses its corresponding power and permanent capacity. |
| Negative material | Graphite negative electrodes inherently have the ability to react with electrolytes. During the binding process, the SEI film of the reaction product is attached to the surface of the electrode, which stops the violent reaction between the electrode and the electrolyte.
However, this reaction also proceeds in a small amount due to the defects of the SEI film. The reaction between the electrolyte and the negative electrode not only consumes the lithium ions in the electrolyte, but also consumes the negative electrode material in the electrolyte. That is to say, the loss of electrical energy generated by the reaction between the electrolyte and the negative electrode will also lead to the loss of the maximum available capacity of the battery. |
| Electrolyte | In addition to reacting with the positive and negative electrodes, it also reacts with the impurities in the positive and negative materials, and even the impurities in the material itself.
These reactions all generate irreversible products leading to the reduction of Li ions, which is responsible for the loss of available capacity. |
- Internal short circuit
In the production process of batteries, some impurities will inevitably be mixed. Some of these impurities can cause a slight conduction of the positive and negative electrodes, which neutralizes the charge and damages the power supply.
In addition, the size deviation and processing burrs of the current collector will also cause the positive and negative electrodes to rotate. Early in the battery's life cycle, it exhibits less self-discharge, but over time there is a greater chance of causing a massive short circuit in the battery.
Therefore, it is necessary to conduct regular battery self-discharge characteristic test experiments under a strict laboratory environment and appropriate humidity level.
- SEI membrane defects
The original function of the SEI film is to isolate the positive and negative electrodes that electrons cannot pass through.If the quality of the film is faulty, it will not work properly, for example, it may cause battery inflation and low voltage. Even small defects can have a significant impact on the self-discharge rate.
And as the battery recycling time continues to increase, the uniformity and density of the SEI film will change. The aging SEI film gradually exposed its shortcomings when protecting the negative electrode, which increased the contact between the negative electrode and the electrolyte and increased the side reactions.
In addition, different qualities of SEI films will also bring about different self-discharge rates during the early use of batteries.
One of the methods to reduce self-discharge is to add additives to improve the quality of SEI film.
(3) Target factor
The self-discharge rate of a battery varies with the application environment, the stage of use, and the state of the application.
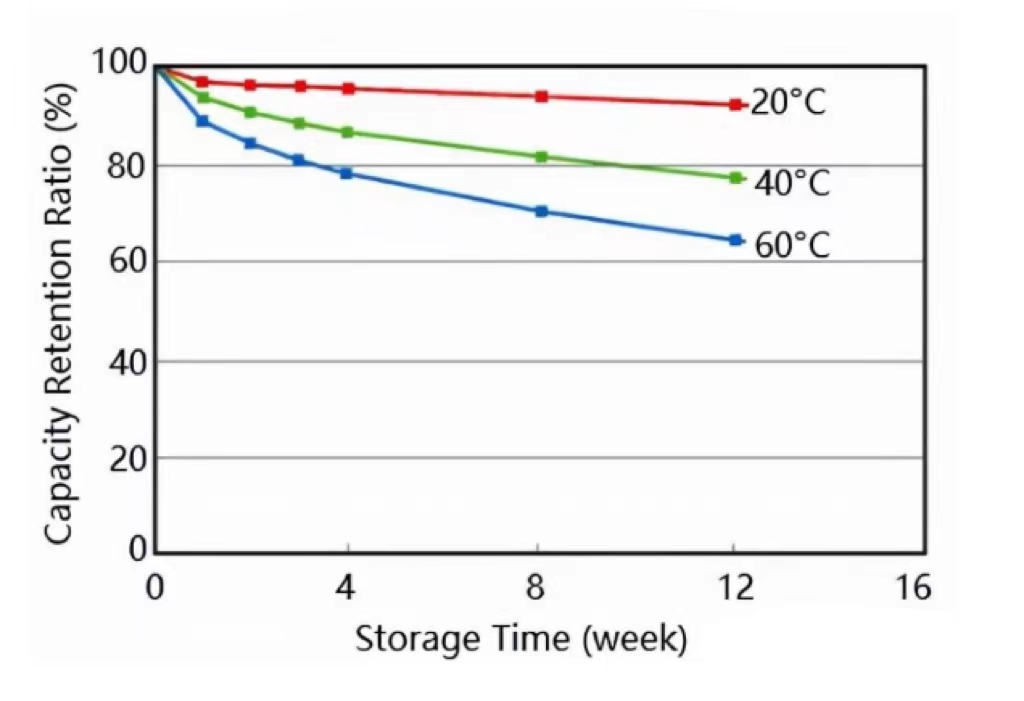
- Temperature
The higher the ambient temperature, the higher the activity of the electrochemical material. The reactions involving the cathode material, anode material, and electrolyte are more intense, causing greater capacity loss at the same time.
- Charge
In particular, the researchers compared the effect of charging on the self-discharge rate. The general trend is that the higher the charge, the higher the self-discharge rate.
In summary, the higher the charge, the higher the positive potential and the lower the negative potential. Therefore, the stronger the oxidizing property of the positive electrode, the stronger the negative polarity, and the stronger the side reaction.
- Time
In the case of the same power and capacity loss, the longer the time, the greater the loss. However, the self-discharge rate is often used as an indicator for comparing different batteries.
That said, under the same premise, time seems to be a factor affecting the degree of self-discharge.
The Purpose of Testing the Self Discharge Rate
The self-discharge rate test has certain reference value.
- Use the self-discharge rate as an indicator of battery quality. Apply it to national standards, compare the product levels of different manufacturers horizontally, and test the quality of the industry.
- For cell sorting. The consistency of the battery pack is an important parameter for the quality of the battery pack after grouping. Various methods have been investigated to group cells and it is expected that cells of the same concentration will be used in the same pack. Self-discharge rate is one of the commonly used indicators for electrostatic screening.
- It can be used as an indicator of product quality control. When testing the same batch of batteries, if some batteries have a high self-discharge rate, indicating that their quality is defective, they must be selected and processed individually.
- Use the self-discharge rate as an index to measure the aging degree of the battery and to evaluate the life cycle of the battery.
Self discharge Detection Method
(1) Voltage drop method
The rate of voltage drop during storage is used to characterize the magnitude of self-discharge. This method is simple to operate, but the disadvantage is that the voltage drop cannot intuitively reflect the loss of capacity.
The voltage drop method is the simplest and most practical, and it is a method commonly used in current production.
(2) Capacity decay method
That is, the percentage of capacity reduction per unit time is expressed.
(3) Self-discharge current method Isd
Calculate the self-discharge current Isd during battery storage according to the relationship between capacity loss and time.
(4) Calculation of Li+ moles consumed by side reactions
Based on the influence of the Li+ consumption rate on the negative electrode SEI film electron conductance during battery storage, the relationship between Li+ consumption and storage time was derived.
Testing the Role of Self Discharge
(1) Prediction problem unit
In the same batch of lithium batteries, the materials and manufacturing process are basically the same.
When the self-discharge of a single cell is obviously too large, it is likely that a serious micro-short circuit is caused by impurities and burrs piercing the separator.
The performance of this kind of battery is not much different from ordinary batteries in the short term, but as the internal irreversible reaction gradually deepens after long-term storage, the performance of the battery will be far lower than its factory performance and other normal battery performance.
The results show that the irreversible loss of the maximum capacity increases significantly (for example, the irreversible capacity loss reaches 5% within 3 months, and the normal battery reaches this value within one year), and the rate capacity retention rate (0.5C/0.2C, 1C/0.2C) decreases, The cycle becomes poor and lithium precipitation occurs.
Therefore, in order to ensure the quality of factory batteries, batteries with large self-discharge must be eliminated.
However, due to the large irreversible capacity loss of self-discharging batteries, the capacity can be regained after at least a quarter of the battery is placed. The battery can be used if the capacity does not decline significantly.
(2) Battery pack
For batteries that need to be assembled, the K value (in the lithium battery industry, refers to the voltage drop of the battery per unit time) is an important indicator.
When the battery pack is assembled into a battery pack, differences in the self-discharge of the cells will cause the cells within the pack to become unbalanced. Self-discharge can also result if there is a leakage current path inside the battery.
Particulate contamination and dendrite growth can create "micro-shorts" inside the battery, creating leakage current paths that can lead to battery failure. Therefore, a battery that self-discharges excessively indicates a possible failure.
In the process of measuring and calculating the K value, since there are obvious differences in the self-discharge levels under different initial voltages, it is necessary to ensure that the primary voltage of the battery is within a small range, and the better primary voltage range is the factory voltage of the battery factory itself.
(3) Help to set the factory voltage and capacity of the battery.
Some customers have such a request that the battery is required to be shipped to the customer at 60% capacity. At this time, it is necessary to evaluate the degree of self-discharge of the battery during transportation to determine the factory voltage or capacity of the battery.
In addition, due to differences in processes, materials, and energy storage stages, this issue requires separate experiments, and data from other experiments cannot be simply applied.
Typical Battery Self Discharge Analysis
Self-discharge is related to the solubility of the positive electrode material in the electrolyte and its instability (easy to self-decompose) after heating. The self-discharge of rechargeable batteries is much higher than that of primary batteries.
Additionally, different battery types have different monthly self-discharge rates. The self-discharge of primary cells is significantly reduced, not exceeding 2% per year at room temperature.
During storage, self-discharge is accompanied by an increase in the internal resistance of the battery, which will lead to a decrease in the load capacity of the battery. In the case of a large discharge current, the energy loss is obvious.
|
Typical battery self-discharge rate |
|
| battery system | Self-Discharge or Lifespan |
| Zinc carbon | 2-3 Years lifespan |
| Lithium metal | 10% in five years |
| Lead acid | 4-6% per month |
| Nickel-cadmium alloy | 15-20% per month |
| Alkaline | 2-3% per month |
| lithium ion | 1-5% per month |
(1) Lithium-ion battery
The self-discharge reactions that occur in Li-ion batteries are very complex. The self-discharge rate of lithium-ion batteries is generally 2%-5% per month, and 5%-8% at room temperature.
When an irreversible reaction occurs inside the battery, the resulting capacity loss is irreversible, mainly including:
Irreversible reaction between cathode material and electrolyte
It mainly occurs in two materials prone to structural defects, such as lithium manganate and lithium nickelate. For example, the reaction of lithium manganate cathode with lithium ions in the electrolyte: liymn2o4xlixe-→lithium xMn2O4.
Irreversible reaction between anode material and electrolyte
The SEI film of lithium-ion batteries is used to protect the negative electrode from electrolyte corrosion. The possible reaction between negative electrode and electrolyte is: LiyC6→Liy-xC6 xLi xe-.
Irreversible reactions caused by impurities in the electrolyte
For example, the possible reaction of CO in two solvents: 2CO2 2e-2Li → lithium carbonate, the reaction of O in two solvents: 1/2O2 2e 2Li. These reactions irreversibly consume lithium ions in the electrolyte, resulting in a loss of battery capacity.
(2) Lead-acid battery
The self-discharge of the lead electrode comes from oxygen evolution and oxygen absorption corrosion. Since the solubility of oxygen in sulfuric acid is small and can be removed, and the concentration of hydrogen ions in the electrolyte is high, the self-discharge phenomenon caused by oxygen evolution is very obvious.
The lead balance electrode has a lower potential than the hydrogen electrode. Due to the high oxygen evolution overpotential of hydrogen, the oxygen evolution reaction is not obvious. If the lead has high purity and less impurities, the oxygen evolution corrosion will be lighter, and the self-discharge will naturally be smaller.
(3) Nickel-cadmium battery
For a fully charged nickel oxide electrode, oxygen evolution reaction (OER) may occur during storage due to the presence of unstable manganese dioxide, resulting in self-discharge.
On the one hand, the negative electrode of cadmium is very stable in the electrolyte, so the self-discharge rate of the nickel-cadmium battery is very small; on the other hand, the electrode surface area of the high-rate discharge nickel-cadmium battery is large, and the self-discharge rate is also large.
(4) Ni-MH battery
Like other batteries, nickel metal hydride batteries are subject to self-discharge. The device of the high-voltage nickel-metal hydride battery fills the entire battery case with hydrogen, and the electroactive material of the negative electrode is in direct contact with the nickel oxide, the electroactive material of the positive electrode, to generate self-discharge during storage.
Summarize
Batteries are subject to self-discharge. This is not a problem of the production line, but the characteristics of the battery.
Although improper manufacturing methods and handling can add to the problem. What we should know is that self-discharge is permanent and cannot be reversed. To reduce self-discharge, it is recommended to store cells and batteries at lower temperatures.
HARVEYPOW - A top lifepo4 battery manufacturer focuses on creating top-notch energy storage batteries, and strictly implements the concept of high standards, high performance, and high environmental protection. Such as our powerwall battery the self-discharge rate is as low as 2% per month.
More FAQ
(1) What causes the battery to discharge?
A short circuit can cause excessive current draw and drain the battery. Check the charging system for loose or worn alternator belts, electrical faults (loose, disconnected, or disconnected wires), or alternator failure.
A malfunctioning engine can also cause the battery to discharge excessively during cranking.
(2) What is battery self-discharge?
Battery self-discharge: completely normal.
Batteries generate electricity through chemical reactions inside the battery, which means that the battery's capacity gradually decreases over time. This phenomenon is called self-discharge. Battery self-discharge cannot be completely avoided. But with proper battery maintenance, the self-discharge of the battery can be reduced.
(3) Which battery has the highest self-discharge rate?
Typical Ni/Cd and Ni/MH batteries have a self-discharge rate of up to 25% per month.
This presents a major logistical problem for users, as NiCd batteries typically need to be charged before they can be used in the field. Lead acid and nickel-cadmium batteries lose their charge very quickly.
(4) How to calculate the battery self-discharge rate?
The most straightforward way: take a battery, charge it, measure its open circuit voltage (OCV), and let it sit without any connections. Going back to the battery later, you will notice that the battery has a lower OCV, indicating that the battery is in a lower state of charge (SoC). This SOC difference is the battery self-discharge rate.
(5) Will the battery lose power when not in use?
In a healthy battery, ions flow freely between the cathode and anode. . . Batteries degrade even when you don't use them. A fully charged lithium-ion battery will lose about 20% of its capacity after a year of normal storage.
