6 Reasons for Lithium Battery Failures & Treatment Measures

Lithium batteries, used in various consumer electronics such as cell phones and laptop computers, can be susceptible to failure. In fact, up to 15% of lithium battery failures are due to manufacturing defects or design flaws. As a result, it is important for consumers to understand the risks associated with these devices and what measures can be taken to avoid potential problems. This article will discuss the causes of lithium battery failures and provide information on available treatments and preventive measures.
The widespread use of lithium batteries has created an ever-increasing need for a better understanding of their operation and limitations. Lithium ion cells are composed of several components that must work together correctly in order for the device to operate safely and efficiently. Unfortunately, if any one component fails or does not perform properly this can lead to instability and potentially catastrophic results. Common causes include overcharging, short-circuiting, overheating, incorrect charging methods and improper handling.
In addition to discussing common causes of lithium battery failure, this article will also cover possible treatment options should a problem arise along with preventative measures that can be taken by consumers. It is essential for users of these products to have access to reliable information so they can make informed decisions about how best to care for their devices. By understanding the risks involved with using lithium batteries as well as taking proper precautions before use, consumers may be able to reduce their risk of experiencing a battery failure incident.
Definition Of A Lithium Battery
A lithium battery is a type of rechargeable, high-energy-density electric power source. It has the ability to store more energy for its size and weight than any other commonly used battery technology. Lithium batteries are typically composed of cells containing electrodes made from a combination of metals such as cobalt or manganese, graphite and lithium salts. The chemical reaction between these elements produces an electrical current when an external circuit connects the two electrodes within the cell.
Lithium batteries have become increasingly popular in recent years due to their lightweight, long life span and excellent performance capabilities. They are widely used in consumer electronics such as laptops, tablets, cameras and smartphones; medical equipment; aerospace applications; electric vehicles; industrial machinery; and military applications.
Types of Lithium Battery Failure
In order to avoid the above-mentioned performance attenuation and battery safety problems, it is imperative to carry out lithium battery failure analysis.
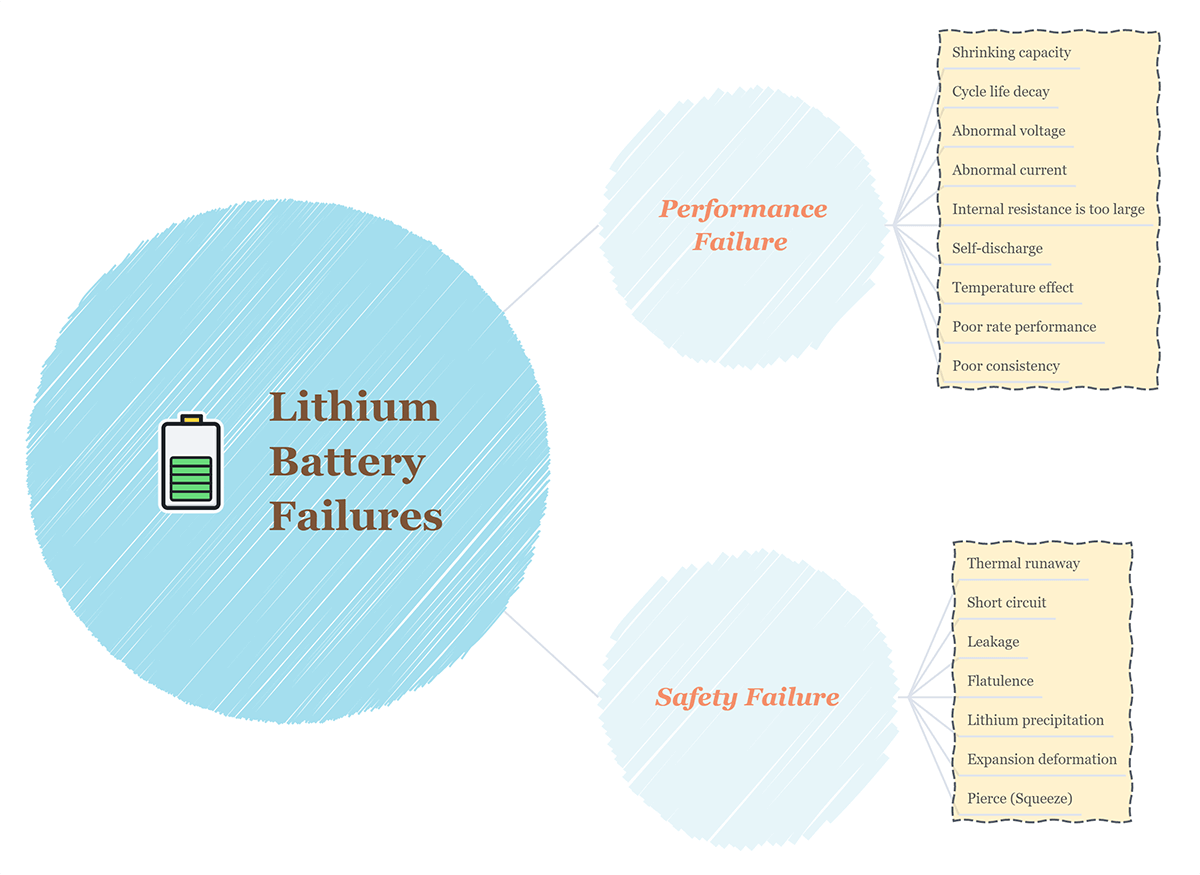
The failure of lithium batteries refers to the attenuation of battery performance or abnormal performance caused by some specific essential reasons, which can be divided into performance failure and safety failure.
Performance Failure: Shrinking capacity, cycle life decay, abnormal voltage, abnormal current, internal resistance is too large, self-discharge, temperature effect, poor rate performance, poor consistency.
Safety Failure: Thermal runaway, short circuit, leakage, flatulence, lithium precipitation, expansion deformation, pierce(Squeeze)
Common Causes and Measures of Lithium Battery Failures
(1) Electrolyte Leakage
Electrolyte leakage is a common lithium battery failure that can occur when the electrolyte comes into contact with moisture. The electrolyte, which is an electrically conducting solution containing ions and free electrons, enables chemical reactions to take place within the cell of the battery. When the electrolyte leaks out of its container due to exposure to too much water or other external factors, it reduces the effectiveness of electrical current flow through the cell, leading to a decrease in performance and capacity.
There are several steps one can take to prevent electrolyte leakage:
- Ensure that batteries are kept dry and away from sources of moisture such as rain or humidity.
- Store them at room temperature for optimal performance and avoid extreme temperatures.
- Inspect for any visible signs of damage before use.
By taking these precautions, users can help protect their batteries from developing issues related to electrolyte leakage. It is also important to regularly monitor performance levels over time in order to recognize changes early on and address any problems quickly before they become more serious. Taking proactive measures will ensure that batteries remain safe and reliable over time.
(2) Internal Short Circuit
When discussing lithium battery failures, internal short circuits are a common cause. This type of failure occurs when the anode and cathode come into contact with each other within the cell. When this happens, electrical current can flow through unintended pathways in the cell which results in overheating and eventually leads to thermal runaway.
Treatment measures for internal short circuit usually involve replacing the faulty component or repairing its connection points. In some cases, it may be necessary to replace the entire cell if it cannot be repaired. Additionally, preventive maintenance is key to avoiding such problems from occurring in the first place. It is important that batteries undergo regular inspections and monitoring so any potential issues can be identified early on and addressed before they become more serious.
(3) Overcharging
Overcharging is an issue that commonly causes lithium battery failure. When a battery is overcharged, the cells become unstable and their chemical composition can change drastically. This instability may cause irreversible damage to the cell components, leading to decreased performance of the battery or even its complete destruction. It is important to remember that all batteries are designed differently, so it is essential to refer to manufacturer instructions if they exist when charging any type of lithium battery.
To prevent overcharging, lithium battery manufacturers often suggest using a specific charger with safety features such as current-limiting and thermal protection. Additionally, users should always ensure that the appropriate voltage for their particular model is applied during charging cycles. Regular maintenance checks should be done on all electronics containing lithium batteries in order to identify any potential issues before an overcharge occurs; this includes checking connections between cells or packs, examining insulation materials for signs of wear and tear, and monitoring charge rates. Taking these steps will help protect against costly repairs and keep lithium batteries performing optimally for longer periods of time.
(4) Manufacturing Defects
As the saying goes, 'Prevention is better than cure.' This adage holds true in the case of lithium battery failures due to manufacturing defects. It is essential for manufacturers to ensure that stringent quality standards are met while producing these batteries. The most common types of manufacturing defects include poor assembly, incorrect size or shape of components and inadequate insulation around electrical connections.
The first step towards preventing such issues is to maintain a high level of cleanliness at all production stages. Apart from this, it is also important to use superior-grade materials when fabricating cells and other parts associated with a battery system. In addition, manufacturers should take extra care in providing adequate insulation around any exposed wires or terminals so as to prevent short circuits or current leakage. Further, every component used during production should be thoroughly tested before being integrated into the final product. By following these basic guidelines, manufacturers can minimize the risk of defective products reaching end-users.
(5) Temperature Change
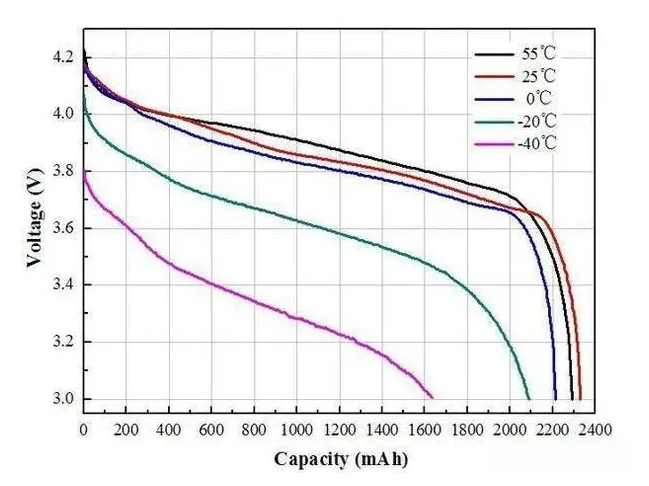
The most common issue associated with temperature change in lithium-ion batteries is reduced capacity or power output. This phenomenon occurs when temperatures become too cold or hot - below freezing point (0°C) or above 45°C respectively.
As the internal resistance increases due to colder temperatures, the current flow decreases and thus reduces overall charge storage capacity; similarly for higher than normal operating temperatures where excessive heat causes accelerated aging resulting in decreased capacity/power output over time. Therefore, it becomes essential to ensure that lithium batteries are operated within safe temperature limits if an optimal performance level is desired.
To protect against adverse effects from extreme temperatures, several approaches have been developed ranging from simple passive methods like using external insulation/cooling systems to active methods such as thermal management systems comprising of thermostats, thermistors etc. which monitor battery temperature constantly and adjust accordingly based on preset parameters.
Implementing such strategies not only helps maintain stable operation even during extreme conditions but also prolongs battery life significantly by protecting cells from damage caused otherwise due to high/low temperatures.
(6)External Damage
External damage to a lithium battery can often be observed visually, such as dents or punctures. If this is the case, then it may have caused an internal short circuit in the cell and exposed its components to the external environment. In some cases, electrical continuity between two terminals of a damaged cell may also occur if contact is made with other metallic objects or conductors. This could lead to thermal runaway and catastrophic failure of the entire battery pack due to excessive heat accumulation.
To prevent such damages from occurring, certain measures should be taken by users of lithium batteries. Careful handling and storage practices must be followed at all times so that external impacts are avoided. Furthermore, protective covers and insulation materials should be used when transporting or storing cells and modules for long periods of time. Finally, appropriate safety protocols should always be implemented when installing packs into devices which might put them at risk of being subjected to mechanical shock or vibration.
Common Fault Performance and Principle Analysis
(1) Capacity fading failure
In the standard cycle life test, the discharge capacity should not be lower than 90% of the initial capacity when the number of cycles reaches 500. Or when the number of cycles reaches 1000, the discharge capacity should not be lower than 80% of the initial capacity.
If the capacity drops sharply within the standard cycle range, it is a capacity fading failure.
The root cause of battery capacity attenuation failure lies in the failure of materials, and is closely related to objective factors such as battery manufacturing process and battery use environment.
From the perspective of materials, the main causes of failure are the structural failure of the positive electrode material, the excessive growth of SEI on the surface of the negative electrode, the decomposition and deterioration of the electrolyte, the corrosion of the current collector, and the trace impurities in the system.
Structural failure of positive electrode materials
Includes particle breakage of positive electrode materials, irreversible phase transition, material disorder, etc.
During the charge and discharge process of LiMn2O4, the structure will be distorted due to the Jahn-Teller effect, and even the particles will be broken, resulting in the failure of the electrical contact between the particles.
The LiMn1.5Ni0.5O4 material will undergo a "tetragonal-cubic" phase transition during the charge and discharge process, and the LiCoO2 material will cause Co to enter the Li layer due to the transition of Li during the charge and discharge process, resulting in a disordered layered structure, and restricting its capacity to play.
Negative electrode material failure
The failure of graphite electrodes mainly occurs on the graphite surface, which reacts with the electrolyte to produce a solid electrolyte interfacial phase (SEI).
If the overgrowth will reduce the content of lithium ions in the internal system of the battery, the result will be capacity fading.
The failure of silicon-based anode materials is mainly due to the cycle performance problems caused by its huge volume expansion.
Electrolyte failure
LiPF6 has poor stability and is easy to decompose, reducing the content of migratable Li+ in the electrolyte.
It also easily reacts with trace amounts of water in the electrolyte to generate HF, causing corrosion inside the battery.
Poor air tightness causes the electrolyte to deteriorate, and the viscosity and chromaticity of the electrolyte change, which eventually leads to a sharp decline in the ion transmission performance.
Failure of current collector
Including corrosion of current collector and decrease of adhesion of current collector.
The HF generated by the failure of the above-mentioned electrolyte will corrode the current collector and form a compound with poor conductivity, resulting in an increase in ohmic contact or failure of the active material.
During the charging and discharging process, the Cu foil is dissolved at a low potential and then deposited on the surface of the positive electrode, which is the so-called "copper precipitation".
The common form of current collector failure is that the binding force between the current collector and the active material is insufficient, resulting in the peeling of the active material, which cannot provide capacity for the battery.
(2) Cycle life decay
Generally speaking, the cycle life of lithium batteries decreases over time, and individual cells begin to degrade, which can eventually lead to complete failure.
However, during use, the charging mode, ambient temperature and aging will all affect the ideal cycle life of lithium batteries.
For example, incorrect charging methods can significantly shorten the life of lithium batteries, and extreme ambient temperatures may increase the self-discharge rate of lithium batteries.
In addition, frequent fast charging and full power storage will also reduce the cycle life of lithium batteries. These can cause the battery to fail before its expected life.
Therefore, monitoring the performance of lithium batteries and complying with correct usage regulations are necessary measures to ensure the ideal life of lithium batteries.
(3) Abnormal voltage
Overvoltage
will lead to excessive charging current, which will cause the battery to heat up, and the higher the current, the faster the temperature rise.
If the battery is overheated for a long time, the inner layer of the battery plate will be undercharged, which will not only greatly reduce the battery storage capacity, but also greatly shorten the life of the battery. In severe cases, the battery will bulge and the top cover will burst.
Undervoltage
- When the lithium battery is packaged, a short-circuit fault is caused between the metal strip and the aluminum-plastic film, so that when it is heated (about 140 ° C) and the aluminum-plastic film is hot-melt and sealed, leakage suddenly occurs.
- Short-circuit faults in the external structure of the lithium battery, such as a large number of batteries knocked down, short-circuit faults where the tabs are overlapped with each other, and the positive and negative tabs of the lithium battery are clamped on the same side when the lithium battery is put on the cabinet.
- Lithium battery internal short-circuit fault, or micro-short-circuit fault, such as: a burr on the positive and negative electrodes touches the short-circuit fault through the separator paper, the separator paper does not wrap the pole piece or is just flush with the pole piece, resulting in a short-circuit failure, etc.
(4) Increased internal resistance
An increase in the internal resistance of a lithium battery will be accompanied by failure problems such as a decrease in energy density, a decrease in voltage and power, and battery heat generation.
The main factors that lead to the increase of the internal resistance of lithium-ion batteries are divided into key materials of the battery and the environment in which the battery is used.
Main battery materials
Including microcracks and fragmentation of positive electrode materials, destruction of negative electrode materials and excessive thickness of SEI on the surface, aging of electrolyte, detachment of active materials from current collectors, poor contact between active materials and conductive additives (including loss of conductive additives), diaphragm shrinkage cavities blocked, abnormal welding of battery tabs, etc.
Battery use environment
High/low ambient temperature, overcharge and overdischarge, high rate charge and discharge, manufacturing process and battery design structure, etc.
(5) Temperature effect
Low-temperature operation
As the temperature drops, the chemical reactions within the battery slow down, resulting in a reduction in current carrying capacity and power handling.
This may make the electrodes unable to withstand excessive current and lead to electroplating of the anode, resulting in irreversible capacity loss.
High-temperature work
Working in high temperatures can be a risky job as it has the potential to cause battery damage.
However, using the Arrhenius effect allows higher power output from the battery cell, but the higher current results in an increased rate of heat dissipation.
The resulting high-temperature levels can lead to thermal runaway if adequate cooling measures are not taken in time.
(6) Consistency problem
During the use of batteries, batteries are often used in series, in parallel or in combination to construct battery packs.
In the construction of a battery pack, when the internal resistance and capacity of the batteries are inconsistent, a battery or a parallel block inside the battery pack will be over-voltage charged and under-voltage discharged during the charging or discharging process. This leads to related safety issues.
(7) Thermal runaway
Thermal runaway means that the local or overall temperature inside the lithium-ion battery rises rapidly, and the heat cannot be dissipated in time, and a large amount of heat accumulates inside, causing the temperature to rise beyond the safety threshold of the lithium-ion battery.
In extreme cases, the temperature of a lithium battery can activate combustible materials in its composition and cause a fire or explosion.
Factors that induce thermal runaway of lithium batteries are abnormal operating conditions, namely abuse, short circuit, high rate, high temperature, extrusion, and acupuncture.
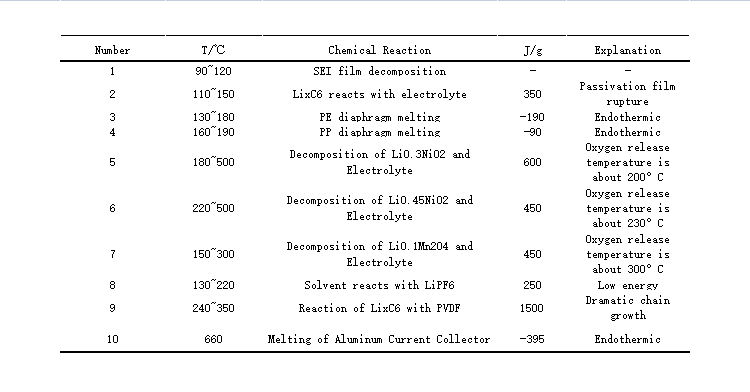
(Heat release from different reactions inside the battery)
(8) Internal short circuit
Internal short circuits often cause self-discharge of lithium-ion batteries, capacity decay, local thermal runaway, and safety accidents.
Short circuit between copper/aluminum current collectors
It is caused by untrimmed metal foreign objects piercing the separator or electrodes during battery production or use, and the displacement of pole pieces or tabs in the battery package, which causes positive and negative current collectors to contact.
Short circuit due to diaphragm failure
Diaphragm aging, diaphragm collapse, diaphragm corrosion, etc. can lead to diaphragm failure.
The failed separator loses its electronic insulation or the gap becomes larger so that the positive and negative electrodes are in micro-contact, and then there will be severe local heating. Continued charging and discharging will spread to the surroundings, resulting in thermal runaway.
Impurities cause short circuit
If the transition metal impurities in the positive electrode slurry are not completely removed, the separator will be pierced or the lithium dendrites in the negative electrode will be formed, resulting in an internal short circuit.
Short circuit caused by lithium dendrites
Lithium dendrites will appear where the local charge is uneven during the long cycle, and the dendrites penetrate the separator and cause an internal short circuit.
In the process of battery design and manufacturing or battery pack assembly, unreasonable design or excessive local pressure will also cause internal short circuit. Under the induction of battery overcharge and overdischarge, internal short circuit will also occur.
(9) Flatulence
The gas production phenomenon that occurs when the electrolyte is consumed to form a stable SEI film during the battery formation process is normal gas production.
However, phenomena such as excessive consumption of electrolyte to release gas or positive electrode material to release oxygen are abnormal outgassing.
Flatulence often occurs in pouch batteries, which will cause excessive internal pressure and deformation of the battery, break the aluminum film of the package, and contact problems with the internal cells.
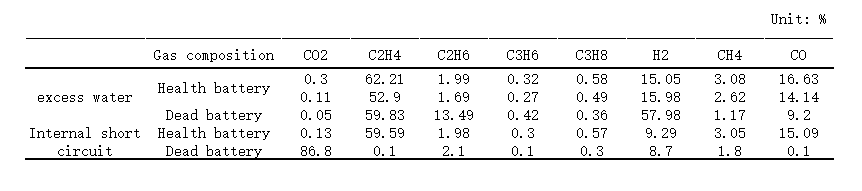
(Gas composition analysis of healthy cells and failed cells)
The trace amount of water in the electrolyte or the electrode active material has not been dried, resulting in the decomposition of lithium salt in the electrolyte to produce HF, which corrodes the current collector Al and destroys the binder to produce hydrogen.
The electrochemical decomposition of chain/cyclic esters or ethers in the electrolyte caused by improper voltage range will produce C2H4, C2H6, C3H6, C3H8, CO2, etc.
(10) Lithium precipitation
Lithium precipitation is the precipitation of metal lithium on the surface of the negative electrode of the battery, which is a common aging failure phenomenon of lithium batteries.
The lithium precipitation will reduce the active lithium ions inside the battery, cause capacity failure, and form dendrites to pierce the separator, resulting in excessive local current and heat, and ultimately leading to battery safety problems.
Where is The Difficulty of Failure Analysis?
Difficulty 1: There is not a simple one-to-one correspondence between failure phenomena and failure causes.
The same failure phenomenon may be caused by different failure causes. Therefore, it is not necessary to use a single failure cause to describe and analyze the failure.
To be correct, it is necessary to analyze the impact weight and primary and secondary relationship of various failure causes at a certain stage from a quantitative perspective, in order to accurately evaluate the failed battery and put forward reasonable measures in a targeted manner.
Difficulty 2: The complex and changeable system composition, preparation process and application environment of lithium batteries have brought challenges to its failure analysis.
The lithium battery itself belongs to the gray box (gray system) in modern cybernetics, that is, its internal physical and chemical change mechanism and thermodynamic and kinetic processes are not completely understood.
In the manufacturing process of lithium batteries, there are many kinds of processes. If a certain parameter design of any process is unreasonable, it will be reflected in the performance of lithium batteries.
4 Steps to Lithium Battery Failure
In summary, we learned that the cause of battery failure is often not caused by a single mistake. Typically, battery failure evolves through 4 stages.
(1) Abuse
A healthy battery fails, usually due to improper use.
Electrical Abuse
Refers to overcharging, short circuits, exceeding the rated discharge current consumption, or even reverse polarity installation.
However, if equipped with a smart BMS to monitor the performance and status of the battery at any time, it can effectively prevent such problems from happening.
This damage can often be prevented with minimal loss and effort by implementing proper protective measures before a major electrical abuse event occurs.
External damage
Refers to causing physical damage to the device, including repeatedly bending or twisting the battery, dropping, puncturing or otherwise damaging the casing containing the lithium battery, etc.
Due to the high reactivity of lithium, all of these types of mechanical damage can have catastrophic consequences.
Under extreme shock or pressure, lithium can release enough energy to start a fire and cause an explosion, reaching temperatures above 1,800°F.
For safety, lithium batteries should always be handled with care and inspected regularly for any signs of external damage before use.

Extreme temperature
Refers to lithium batteries exposed to high temperature or cold environments for a long time.
Lithium-ion batteries can be prone to failure if stored at temperatures above a relatively moderate 32°C (90°F) or below 0°C (32°F).
A battery that is too hot will experience a gradual loss of capacity, while a battery that is too cold can cause a "hard failure" that prevents it from starting properly.
To prevent such problems, lithium batteries should ideally be stored in a climate-controlled environment optimized for battery safety and longevity.
(2) Flatulence
Lithium batteries are commonly used to store energy due to their high capacity and reliability.
Outgassing occurs when lithium ions move from the anode of a lithium battery during charging and release gas, causing an increase in pressure within the battery. The pressure can become so great that it can cause the lithium battery's vents and covers to come off or even crack.
This outgassing can lead to catastrophic events such as fire or explosion if lithium batteries are overcharged or exposed to temperatures beyond their thermal limits.
(3) Smoke generation
If smoke starts to develop, it indicates thermal runaway, a stage where the battery can quickly progress to a dangerous state.
There is a high probability that it will cause fire or material rupture, and eventually the battery will fail. This thermal threat can easily spread to other nearby equipment if precautions are not taken immediately.

(4) Fire
When a battery cell reaches thermal runaway, it can generate its own energy and add heat while burning. So from stage 3 and stage 4 usually happens in close succession.
In addition, due to the presence of metal oxide electrodes in lithium-ion batteries, which generate oxygen during the decomposition process, fires can quickly spread to multiple cells in a battery pack, resulting in a huge hazardous event.
How To Replace A Damaged Battery?
Replacing a damaged battery can be a difficult task. However, with the right tools and know-how, this process can easily be completed in a safe manner.
- First, it is important to ensure that the area around the damaged battery is clear of obstructions and flammable materials.
- Once this has been done, disconnect any cables or wires connected to the battery before removing it from its housing.
- The next step involves taking off any protective covering on the new battery before inserting it into its designated space.
- After securely fastening all connections and securing them with clamps if necessary, inspect for signs of leakage or corrosion before replacing the cover over the connection points.
- Finally, testing should be carried out to make sure everything is functioning correctly.
Causes Of Lithium-Ion Batteries Catching Fire

Lithium-ion batteries are widely used in many consumer electronics, however, the risk of them catching fire is a severe safety concern.
Though there could be several other factors contributing to these failures, the most common ones include:
- Short circuits due to internal or external faults result in high current flow leading to excessive heating and thermal runaway.
- Overcharging or overheating caused by inadequate temperature control systems can cause irreversible breakdowns within components.
- Mechanical damages such as punctures or crushing can also disrupt normal functioning.
- Lack of adequate ventilation inside device enclosures can reduce air circulation thereby increasing temperatures.
All these issues can ultimately lead to degradation in performance characteristics along with lithiation phenomena i.e., deposition of lithium metal onto cell surfaces, thus making them highly dangerous if left unattended. It is therefore essential that proper preventive measures are taken while designing any device that makes use of lithium-ion batteries so as not only to ensure their safe usage but also to prevent any catastrophic mishaps due to malfunctioning cells.
What Should We Do If the Lithium Battery Catches Fire?
If the lithium battery catches fire, use a dry powder fire extinguisher or carbon dioxide fire extinguisher to extinguish the fire immediately.
The dry powder fire extinguisher is equipped with a dry powder fire extinguishing agent such as ammonium phosphate salt inside, and it is easy to flow and dry. It is composed of inorganic salt and crushed and dried additives, which can fully and effectively extinguish the fire in the event of a fire.
The carbon dioxide fire extinguisher uses the filled liquid carbon dioxide substance to spray out to extinguish the fire.
Precautions for fire fighting
- First cut off the power supply, use the nozzle of the fire extinguisher to aim at the root of the flame and spray to achieve the purpose of extinguishing the fire.
- It is not recommended to use water to extinguish the lithium battery, because there may be live equipment around, and there are certain safety hazards in extinguishing fires with water. If you don't have a fire extinguisher, you can also use sand to extinguish the fire.
- People should not stand too close when fighting the fire. If the fire is found to be uncontrollable, immediately notify the surrounding people to evacuate to a safe place to avoid explosion after the lithium battery burns.
- Lithium batteries will produce toxic gas when they catch fire. Therefore, it is necessary to wear a protective mask to prevent poisoning from inhaling toxic gases.
- When the fire is too large, you should call the fire department in time, because it is not ruled out that there are flammable and explosive materials around the fire.

Learn More Top Questions About Lithium Batteries!
Choose the Best Safety Battery
So, as we've seen in this blog post, lithium battery failures can be complex and difficult to diagnose.
We urge everyone to take extreme care when handling lithium batteries, as the risk of fire and other hazards remains high if proper safety procedures are not considered.
With the development of technology, we have ushered in the lifepo4 battery, which is currently the safest lithium battery in the world, with a high ignition point and almost no fire or explosion.
HARVEYPOW lifepo4 battery manufacturer is committed to improving battery performance on the premise of ensuring battery environmental protection and safety. 8000 times cycle life and IP65 waterproof grade allows them to confidently provide a 12-YEAR WARRANTY.
Each lifepo4 battery pack is equipped with smart BMS and Bluetooth, which can monitor battery performance and status at any time to prevent battery overcharge, over-discharge, short circuits and other problems.
With a good battery, every family is worth it!
